Uncover Response Rates Through Week 121,2
 Indicates visits at which STELARA® was administered to patients randomized to receive STELARA® at baseline.
Indicates visits at which STELARA® was administered to patients randomized to receive STELARA® at baseline.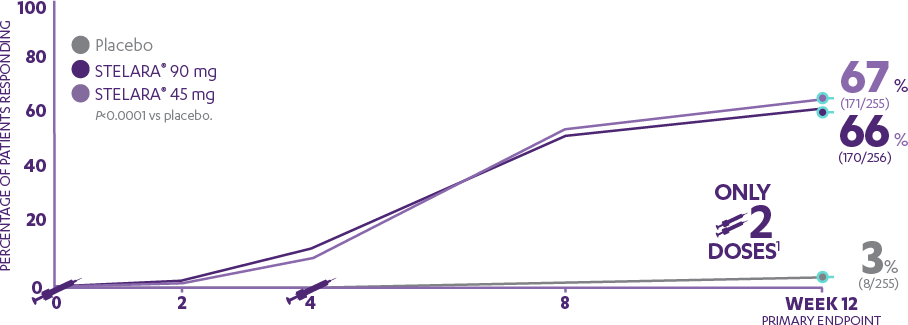
IN PHOENIX 1:
IN PHOENIX 2:
PGA 0/1 was a major secondary endpoint at Week 12, and was achieved by 7 of 10 patients in the 45-mg and 90-mg groups (68% [277/409] and 73% [300/411], respectively) compared with 4% (18/410) in the placebo group (P<0.0001 vs placebo for each dose).1,3
 Indicates visits at which STELARA® was administered to patients randomized to receive STELARA® at baseline.
Indicates visits at which STELARA® was administered to patients randomized to receive STELARA® at baseline.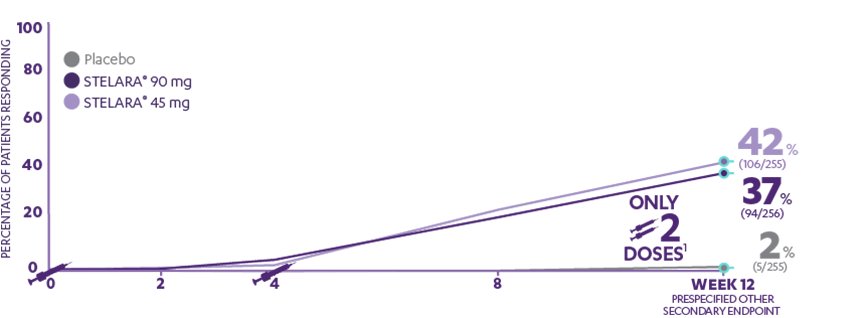
IN PHOENIX 2:
IN PHOENIX 1:
IN PHOENIX 2:
PGA 0/1 was a major secondary endpoint at Week 12, and was achieved by 7 of 10 patients in the 45-mg and 90-mg groups (68% [277/409] and 73% [300/411], respectively) compared with 4% (18/410) in the placebo group (P<0.0001 vs placebo for each dose).1,3
90 mg (94/256) achieved PASI 90 response
IN PHOENIX 2:
In adults with moderate to severe plaque psoriasis
Discover Consistent Response Rates Over Time
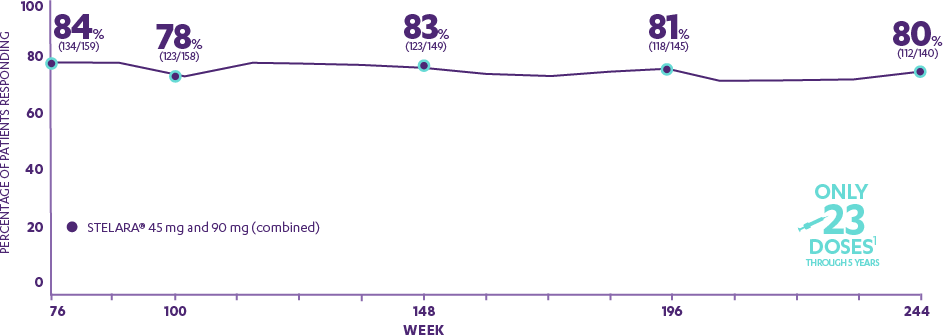
Patients who were rerandomized to continue every-12-week dosing after responding to STELARA® at Weeks 28 and 40
- 84%
- 78%
- 83%
- 81%
- 80%
- WEEK 76
- WEEK 100
- WEEK 148
- WEEK 196
- WEEK 244
Through 5 Years
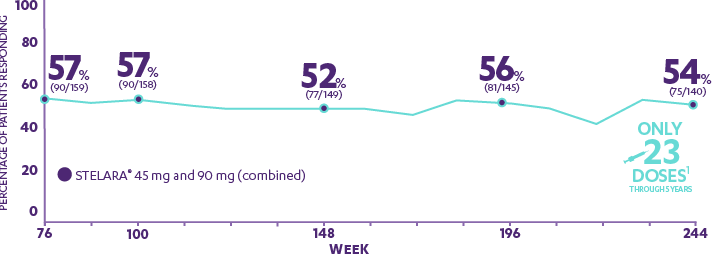
Patients who were rerandomized to every-12-week dosing after responding to STELARA® at Weeks 28 and 40
- 57%
- 57%
- 52%
- 56%
- 54%
- WEEK 76
- WEEK 100
- WEEK 148
- WEEK 196
- WEEK 244
Through 5 Years
References: 1. STELARA® (ustekinumab) [package insert]. Horsham, PA: Janssen Biotech, Inc. 2. Leonardi CL, Kimball AB, Papp KA, et al; for the PHOENIX 1 study investigators. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet. 2008;371(9625):1665-1674. 3. Papp KA, Langley RG, Lebwohl M, et al; for the PHOENIX 2 study investigators. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371(9625):1675-1684. 4. Data on file. Janssen Biotech, Inc. 5. Kimball AB, Papp KA, Wasfi Y, et al; on behalf of the PHOENIX 1 investigators. Long-term efficacy of ustekinumab in patients with moderate-to-severe psoriasis treated for up to 5 years in the PHOENIX 1 study. J Eur Acad Dermatol Venereol. 2013;27(12):1535-1545.
