IN ACTIVE PSORIATIC ARTHRITIS
A CHOICE OF ADMINISTRATION OPTIONS FOR
A CHOICE OF ADMINISTRATION
OPTIONS FOR
STELARA® (ustekinumab)
Dosing
- The recommended dose is 45 mg initially and 4 weeks later, followed by 45 mg every 12 weeks1
- For patients with coexistent moderate to severe plaque psoriasis and weighing >220 lbs, the recommended dose is 90 mg initially and 4 weekslater, followed by 90 mg every 12 weeks1
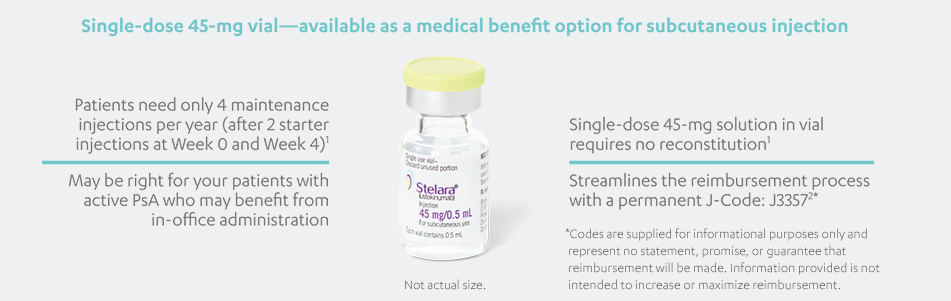

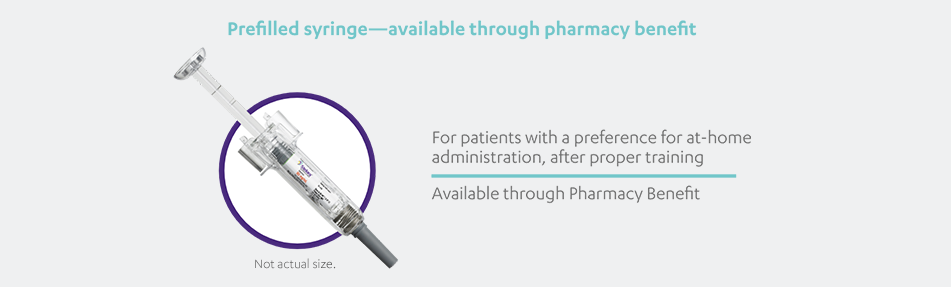
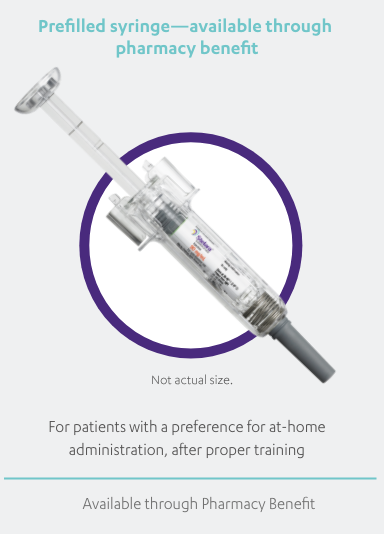
STELARA®, available as 45 mg and 90 mg prefilled syringe and 45 mg single-dose vial, is a subcutaneous injection intended for use under the
guidance and supervision of a physician with patients who will be closely monitored and have regular follow-up visits. Patients may self-inject
with STELARA® after physician approval and proper training. Patients should be instructed to follow the direction provided in the Medication
Guide.


IN ADULT PATIENTS WITH ACTIVE PSORIATIC ARTHRITIS
FEWER INJECTIONS PER YEAR THAN OTHER BIOLOGIC
BIOLOGIC THERAPIES FOR PsA
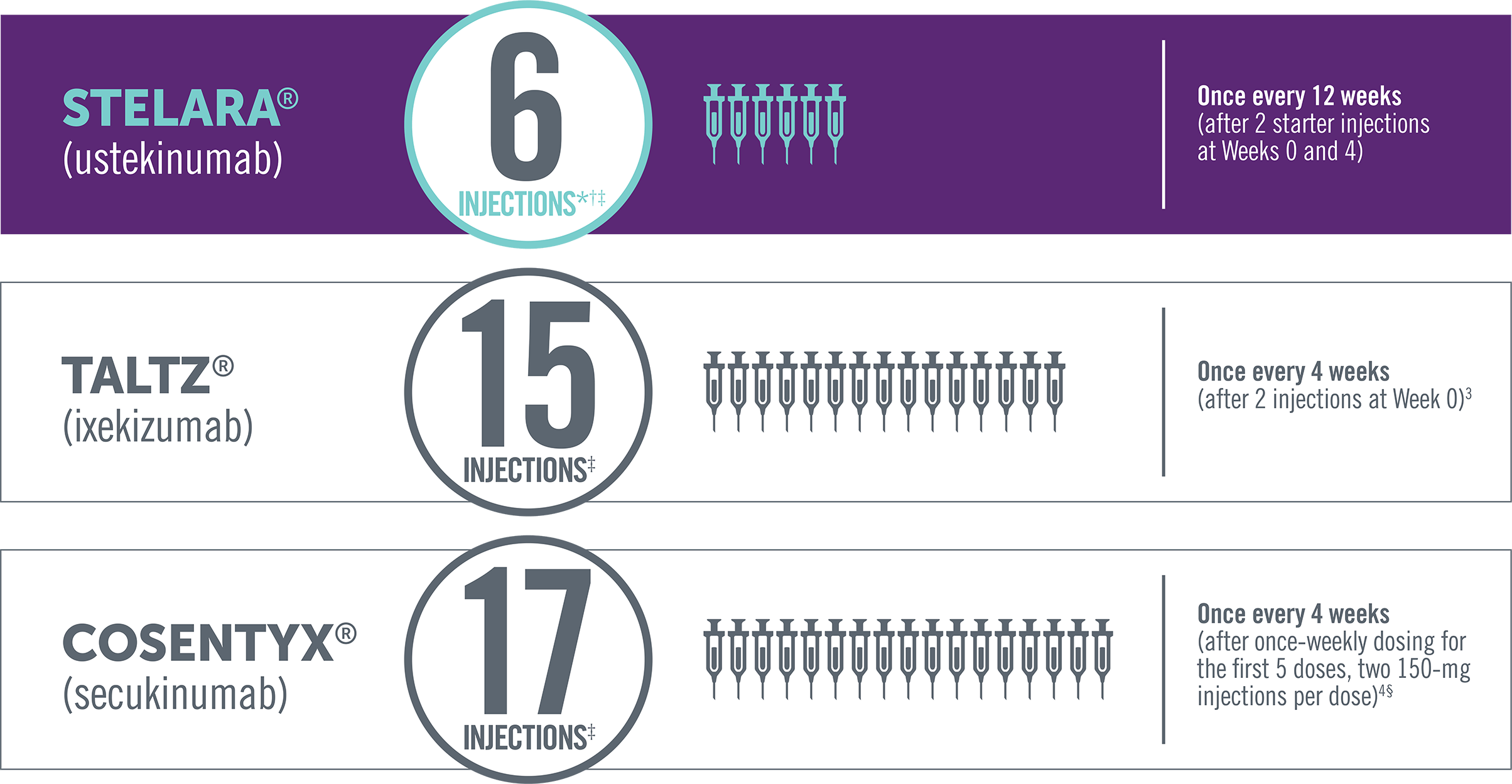
Dosing of subcutaneous non-TNF biologic treatments in PsA
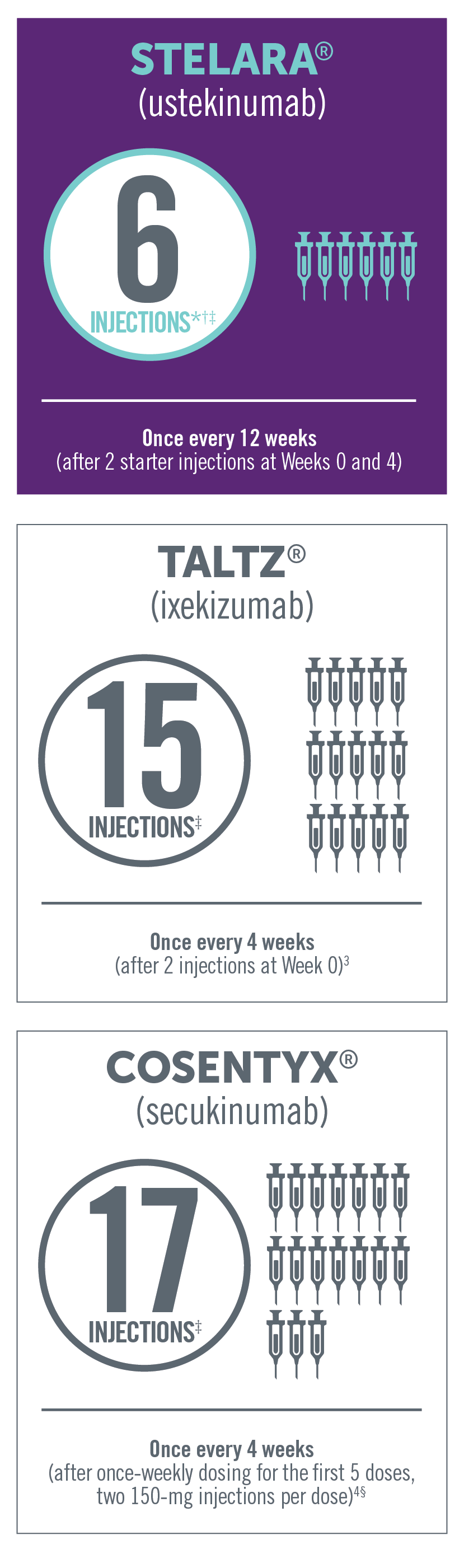
Product comparisons with regard to efficacy and safety cannot be made in the absence of head-to-head clinical studies. This
presentation is not intended to compare the relative safety or efficacy of these treatments. Please refer to the full Prescribing
Information of each agent for dosage and administration.
Taltz is a registered trademark owned or licensed by Eli Lilly and Company, its subsidiaries, or affiliates.
Cosentyx is a registered trademark of Novartis AG.
*Four injections per year—once every 12 weeks after starter injections, in the first year, at Weeks 0 and 4.
†In a patient >220 lb, the recommended dose for STELARA® is 90 mg initially and 4 weeks later, followed by 90 mg every 12 weeks. In this case, the
number of injections per year would be 12.
‡Total number of injections in the first 52 weeks of therapy.
§For patients with PsA with coexistent moderate to severe plaque psoriasis, use the dosing and administration recommendations for plaque
psoriasis. For other patients with PsA, administer Cosentyx® with or without a loading dose by subQ injection. The recommended dosage:
- With a loading dose is 150 mg at Weeks 0, 1, 2, 3, and 4 and every 4 weeks thereafter
- Without a loading dose is 150 mg every 4 weeks
- If a patient continues to have active PsA, consider a dosage of 300 mg every 4 weeks. Cosentyx® may be administered with or without MTX
Product Indications
Taltz® (ixekizumab) is indicated for the treatment of adults with active PsA.3
Cosentyx® (secukinumab) is indicated for the treatment of adult patients with active PsA.4
Cosentyx is a registered trademark of Novartis AG.
Taltz is a registered trademark owned or licensed by Eli Lilly and Company, its subsidiaries, or affiliates.
References: 1. STELARA® [Prescribing Information]. Horsham, PA: Janssen Pharmaceuticals, Inc. 2. Data on file. Janssen Biotech, Inc. 3. Taltz® [package insert]. Indianapolis,
IN: Eli Lilly and Company, 2019. 4. Cosentyx® [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corp; 2018.